U.S. Drug Pricing Policy Shifts: Strategic Implications for Pharma Leaders
Published on 19 Sept 2025
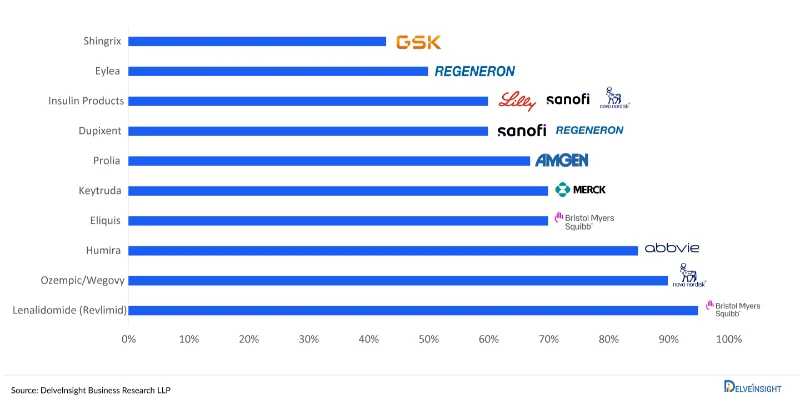
The US administration’s May 12, 2025 executive order established a pricing benchmark that could slash pharmaceutical costs by 40-90% for select high-cost therapies. The Department of Health and Human Services will now benchmark US drug prices against the lowest prices paid by OECD countries with GDP per capita exceeding 60% of US levels, encompassing over 20 countries from Lithuania to South Korea.
As we approach the September 29 compliance deadline, pharma industry executives face the most aggressive pricing intervention in US pharmaceutical history, one that threatens to fundamentally reshape global drug economics.
This whitepaper breaks down the critical implications of the Most Favored Nation (MFN) policy and the looming threat of steep, unprecedented tariffs reaching 250%, translating complex policy changes into actionable intelligence for strategic decision-making.
DelveInsight’s analysis projected $200-300 billion in annual revenue at risk across the US pharmaceutical sector, with the timing particularly precarious as major companies enter a patent cliff period where $150+ billion in revenue faces generic competition between 2025-2030.
As the pharmaceutical industry approaches the compliance deadline, two scenarios emerge: transformative compliance or prolonged legal confrontation. The administration's willingness to deploy regulatory penalties and escalating tariffs suggests sustained pressure regardless of initial industry resistance.
The $300 billion question remains whether this pricing revolution will achieve its stated goals of patient affordability and innovation preservation, or trigger the industry disruption critics warn could undermine American pharmaceutical leadership on the global stage.
Download our comprehensive whitepaper "U.S. Drug Pricing Policy Shifts: Strategic Implications for Pharma Leaders" for detailed analysis and strategic recommendations.
